Fission weapons discussed above are ultimately limited in their destructive capability by the sheer size a subcritical mass can assume -- and be imploded quickly enough by high explosives to form a supercritical assembly. The largest known pure fission weapon tested had a 500 kiloton yield. This is some thirty-eight times the release which destroyed Hiroshima in 1945. Not satisfied that this was powerful enough, designers developed thermonuclear (fusion) weapons.
Fusion exploits the energy released in the fusing of two atoms to form a new element; e.g. deuterium atoms fusing to form helium, 2H + 2H = 4He2 , as occurs on the sun. For atoms to fuse, very high temperatures and pressures are required. Only fusion of the lightest element, hydrogen, has proven practical. And only the heavy isotopes of hydrogen, 2H (deuterium) and 3H (tritium), have a low enough threshold for fusion to have been used in weapons successfully thus far.
The first method tried (boosting) involved simply placing 3H in a void within the center of a fission weapon, where tremendous temperatures and high pressures were attendant to the fission explosion. This worked; contributing energy to the overall explosion, and boosting the efficiency of the Pu fissioning as well (fusion reactions also release neutrons, but with much higher energy).
Because 3H is a gas at room temperature, it can be easily 'bled' into the central cavity from a storage bottle prior to an explosion, and impact the final yield of the device. This is still used today, and allows for what is termed 'dial-a-yield' capability on many stockpiled weapons.
Multistage thermonuclear weapons -- the main component of today's strategic nuclear forces -- are more complex. These employ a 'primary' fission weapon to serve merely as a trigger. As mentioned above, the fission weapon is characterized by a tremendous energy release in a small space over a short period of time. As a result, a very large fraction of the initial energy release is in the form of thermal X-rays.
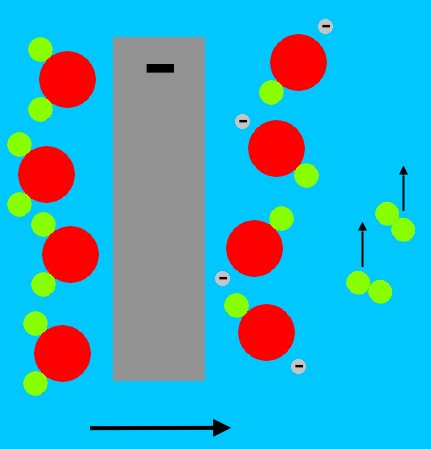
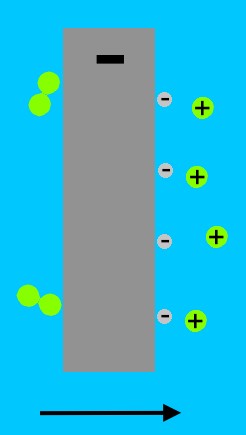
These X-rays are channeled to a 'secondary' fusion package. The X-rays travel into a cavity within a  cylindrical radiation container.
cylindrical radiation container.
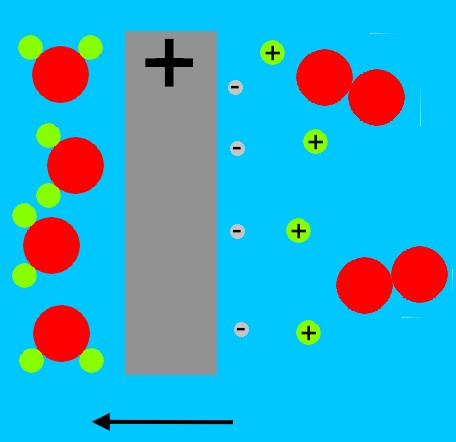
The radiation pressure from these X-rays either directly, or through an intermediate material often cited as a polystyrene foam, ablates a cylindrical enclosure containing thermonuclear fuel (shown in blue at left); this can be Li2H (lithium deuteride).
Running along the central axis of this fuel is a rod of fissile material, termed a 'sparkplug'.
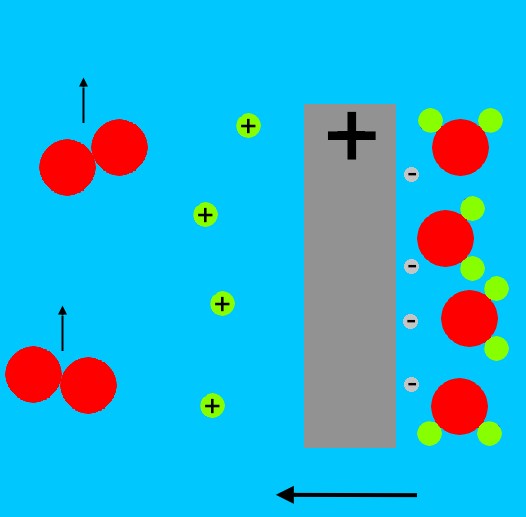
The contracting fuel package becomes denser, the sparkplug begins to fission, neutrons from this transmute the Li2H into 3H that can readily fuse with 2H (the fusion reaction 3H + 2H has a very high cross-section, or probability, in typical secondary designs), heat increases greatly, and fusion continues through the fuel mass.
A final 'tertiary' stage can be added to this in the form of an exterior blanket of 238U, wrapping the outer surface of the radiation case or the fuel package. 238U is not fissionable by the slower neutrons which dominate the fission weapon environment, but fusion releases copious high energy neutrons and this can fast fission the ordinary uranium.
This is a cheap (and radiologically very dirty) way to greatly increase yield. The largest weapon ever detonated -- the Soviet Union's 'super bomb', was some 60 MT in yield, and would have been nearer 100MT had this technique been used in its tertiary. Again, to control the yield precisely, 3H may be bled from a separate tank into the core of the primary, as shown in the hypothetical diagram on the left of a modern thermonuclear weapon.
This primary/secondary/tertiary or multistage arrangement can be increased -- unlike the fission weapon -- to provide insane governments with any arbitrarily large yield.
Fusion, or thermonuclear weapons, are not simple to design nor are they likely targets of construction for would-be terrorists today.
Many aspects of the relevant radiation transport, X-ray opacities, and ultra-high T and D equations-of-state (EOS) for relevant materials are still classified to this day (though increasing dissemination of weapons-adaptable information from the inertially-confined fusion (ICF) area may change this in time). Keeping such information classified makes good sense.
Source: Simpelthiniking.com